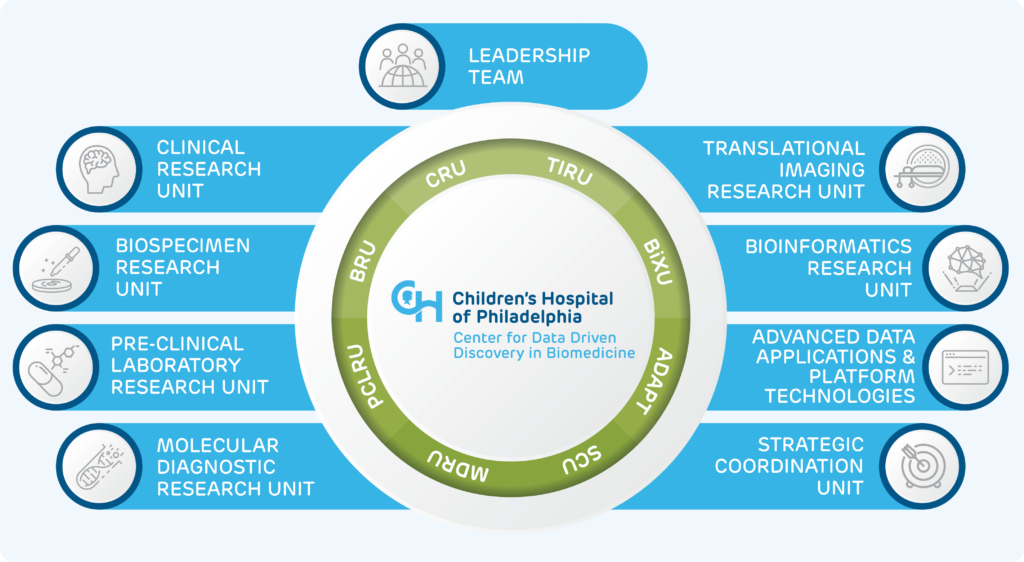
D3b Center Unit Organization
Biospecimen Clinical Research Unit (BCRU)
The Biospecimen Clinical Research Unit (BCRU) supports observational and interventional trials including the management of subject enrollment and consent, regulatory requirements, clinical data collection, and biospecimens. BCRU is responsible for the standardized collection, processing, management and distribution of samples for research and discovery. The BCRU supports the Department of Surgery’s partnered research across the rare disease space, with a specific focus in pediatric brain tumors, epilepsy and fetal birth defects such as craniofacial abnormalities. Additionally, BCRU collaborates with the CHOP Biorepository Core and Department of Pathology.
Pre-clinical Laboratory Research Unit (PCLRU)
The Preclinical Laboratory Research Unit (PCLRU) supports the D³b Center’s basic and translational research efforts. The PCLRU focuses its research on pediatric central nervous system and convergence research in Neuroscience. The primary goal of the PCLRU unit is to harness data-driven approaches on behalf of precision-based and targeted therapeutics using translational methodologies, including projects focused on the elucidation of cell signaling mechanisms of childhood development, oncogenesis, tumor progression, the creation and characterization of preclinical models, drug screening, development and testing.
Molecular Diagnostic Research Unit (MDRU)
The Molecular Diagnostic Research Unit (MDRU) is directly partnered with the Department of Pathology’s Division of Genomic Diagnostics, under the leadership of Marylin Li, MD. The MDRU focuses on the research and development of innovative clinical platforms and tests. The MDRU concentrates its efforts on the clinical harnessing of multimodal molecular data generation and analysis of biomarkers for direct clinical application. The MDRU works to develop new methods and workflows to improve and optimize disease prognosis, clinical diagnostic effectiveness and specificity and patient risk and treatment stratification.
Translational Imaging Research Unit (TIRU)
The Translational Imaging Research Unit (TIRU) uses experimental imaging techniques to measure the progress of children in brain tumor trials, much like those used for tissue biopsy in leukemia and other pediatric cancer trials. The TIRU is using molecular imaging scans (positron emission tomography of PET) to measure tumor cell activity (metabolism) immediately after treatment, or when standard MRI is not conclusive.
Bioinformatics Research Unit (BiXU)
The Bioinformatics Research Unit (BiXU) is composed of bioinformatics engineers and bioinformatics scientists who build bioinformatics pipelines, write analytic code and provide genetic insights to harmonize, analyze and interpret genomics data.
Advanced Data Applications & Platform Technologies Unit (ADAPT)
The Advanced Data Applications & Platform Technologies Unit (ADAPT) members are responsible for designing, building and operating software platforms and infrastructure for the management, distribution and scalable analysis of data.
Clinical and Translational Data Science Unit (CTDSU)
The Clinical and Translational Data Science Unit (CTDSU) leverages clinical, real-world, and high-dimensional molecular data generated by the Children’s Brain Tumor Network (CBTN) to drive data-driven decision-making as part of precision medicine molecular tumor boards for childhood brain cancers. CTDSU works directly with physicians participating in the Pacific Pediatric Neuro-Oncology Consortium (PNOC), the National Cancer Institute’s (NCI) Childhood Cancer Data Initiative, and CHOP’s Frontier precision medicine initiative to support evidence-based therapy recommendations. CTDSU is composed of translational data scientists and biostatisticians who develop clinicogenomic workflows and analytical methods to support clinical decision making.
Strategic Coordination Unit (SCU)
The Strategic Coordination Unit (SCU) provides support for communication, creative services, financial and grant management, program and project management, strategic planning, operational infrastructure development, human resources and team development. The SCU additionally coordinates the execution of over 80 material transfer agreements, data disclosures, data use agreements, contracts and agreements.